Introduction
Acute Graft-versus-host disease (aGvHD) is a major source of mortality following allogeneic hematopoietic cell transplantation (allo-HCT). Fecal microbiotherapy has shown promising results in several pilot studies in patients with refractory gastrointestinal (GI)-aGvHD. Here we report clinical outcomes from 111 patients diagnosed with steroid-refractory (SR) or dependent (SD) GI-aGvHD treated with the pooled allogeneic microbiotherapy MaaT013 as part of the Early Access Program (EAP) in Europe.
Patients and methods
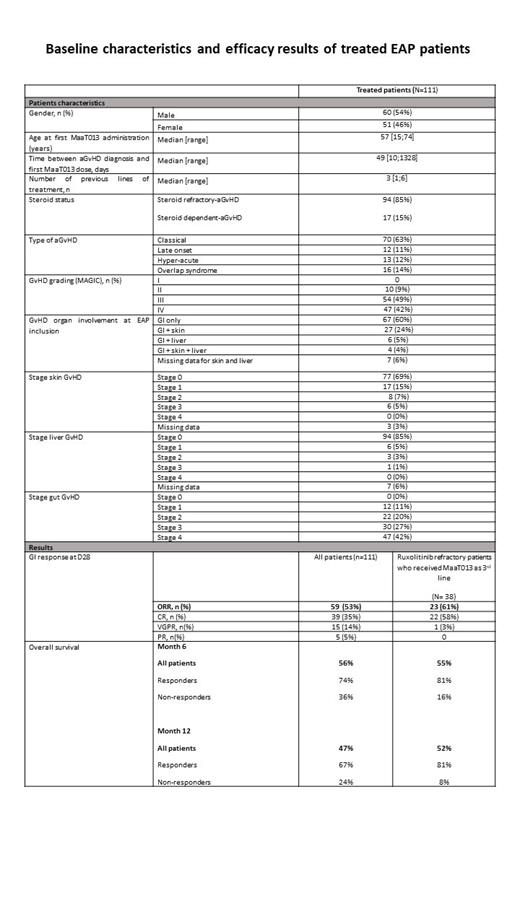
One hundred and eleven patients (including 1 pediatric patient aged 15 years) with SR/SD GI-aGvHD (Classical n=70, late onset n=12, overlap syndrome n=16, hyper-acute n=13) were treated with MaaT013 therapy as part of Early Access Program in Europe (France and Germany). These patients had previously failed 1 to 6 systemic GvHD treatment lines (median 3; 94/111 received ruxolitinib). Most patients had grade III to IV aGvHD (9% grade II, 49% grade III aGvHD, 42% grade IV).
For each patient, a total of 3 MaaT013 administrations were planned every 7 +/- 2 days (median dose administered 3). Each dose is composed of 30 g of feces in 150 mL of suspension from 4 to 8 healthy donors administered by enema (except for 1 patient by nasogastric tube).
Treatment response was calculated among all treated patients based on aGvHD staging and grading at day 28 (D28) at the time of the EAP request.
Results
At D28, the GI-ORR was 53%: 39 CR (35%), 15 VGPR (14%), 5 PR (5%). GI-ORR was higher in patients with lower grade aGvHD (100% in grade II, 63% in grade III, 32% in grade IV) and higher in SD versus SR (88% versus 47%). Overall response considering all organs (n=108 with 3 missing data) was 50%, including 34 CR, 16 VGPR and 4 PR.
Administration of MaaT013 in the pediatric patient was well tolerated and led to complete response of GI and skin symptoms (the patient had stage 3 skin and stage 4 GI aGvHD) up to 12 months. Symptoms of the liver were not resolved at D28 (stage 2 liver, stable disease) but improved from month 6 without additional therapy.
Overall survival (OS) was 56% at 6 months (M6) and 47% at M12 . The median follow-up among surviving patients was 355 days (range, 27-731).OS was significantly higher in patients achieving at least GI-PR at D28 (Responder, R; n=59) compared to patients in treatment failure (Non-responder, NR; n=52): 74% versus 36% at M6, 67% versus 24% at M12 (p<0.0001 log-rank test) . Median survival duration in R was 293 days versus 56 days in NR.
Interestingly, in the subgroup of 38 patients previously treated with ruxolitinib as 2 nd line and MaaT013 as 3 rd line GI-ORR was improved being 61% D28, with 58% CR. OS at M6 was 55% and 52% at M12. OS was significantly higher in R patients, when compared to NR patients (81% versus 16% at M6 and 81% versus 8% at M12 for R and NR respectively, p<0.0001 log-rank test).
MaaT013 displays a good overall safety profile in EAP population: 29 pharmacovigilance cases were reported in 27 patients, including 18 cases that could be possibly considered related to MaaT013 either by the physician or the company: sepsis in 5 patients, bacteremia in 7 patients, rectal bleeding/ anorectal disorder in 3, C. difficile colitis in 1, E. coli osteoarthritis in 1, detection of G. silvicola in stools in 1. No pathogen transmission has been reported. In 2 patients, non-pathogenic commensal bacteria isolated following infectious events were detected in the administered MaaT013 batch. Causality could not be formally excluded in these cases.
56 deaths have been reported. The cause of death was GvHD in 23 patients, severe infection in 15, relapse of underlying malignancy in 9, COVID-19 in 5, hemorrhage during surgery in 1, neurological complications post allo-HCT in 1, and cardiac arrest in 2 patients. No causality link with MaaT013 administration has been identified.
Conclusion
Overall, EAP clinical data showed that MaaT013 was safe and effective for the treatment of SR/SD-GI-aGvHD especially in patients having previously received ruxolitinib. Interestingly, response of GI-aGvHD correlates with increased overall survival, suggesting a strong favorable benefit-risk profile for MaaT013. A Phase 3 trial is currently ongoing to confirm these results in ruxolitinib-refractory patients (NCT04769895).
Disclosures
Malard:Therakos/Mallinckrodt: Honoraria; Sanofi: Honoraria; JAZZ Pharmaceuticals: Honoraria; Gilead: Honoraria; Novartis: Honoraria; Bristol Myers Squibb: Honoraria. Loschi:Abbvie: Honoraria; Alexion: Honoraria; Astra Zeneca: Honoraria; BMS Celgene: Honoraria; Gilead: Honoraria; GSK: Honoraria; Jazz: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Sobi: Honoraria; Takeda: Honoraria. Cluzeau:Servier: Consultancy, Speakers Bureau; Incyte: Speakers Bureau; Keros: Speakers Bureau; Syros: Speakers Bureau; Jazz Pharma: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Huynh:Medac: Other: Advisory board; Astellas: Other: Advisory board; Servier: Other: Advisory board; Pfizer: Other: advisory board; Jazz: Other: travel fees, advisory board; Novartis: Other: travel fees, advisory board; Neovii: Other: Advisory board. Camus:Kite-Gilead: Honoraria. Chevallier:Mallinckrodt Pharmaceuticals: Honoraria; Sanofi: Honoraria; Incyte: Honoraria, Research Funding; Takeda: Honoraria; Immedica Pharma: Honoraria; Servier: Honoraria. Mediavilla:AbbVie: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria. Klein:Novartis: Honoraria. Bruelle:MaaT Pharma: Current Employment. Plantamura:MaaT Pharma: Current Employment, Current holder of stock options in a privately-held company. Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal